BEST SELLERS
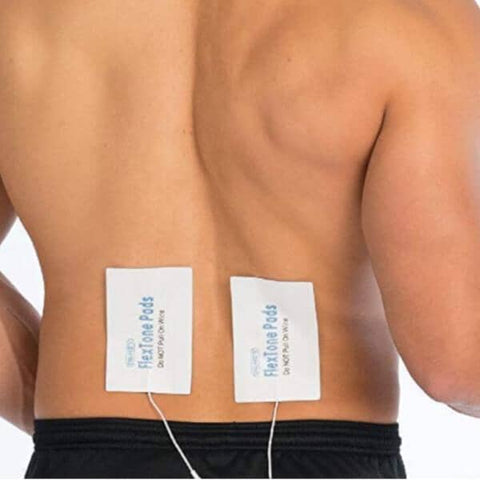
REPLACEMENT GEL PADS
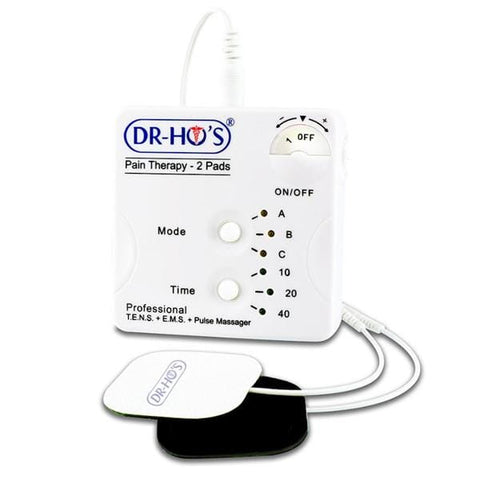
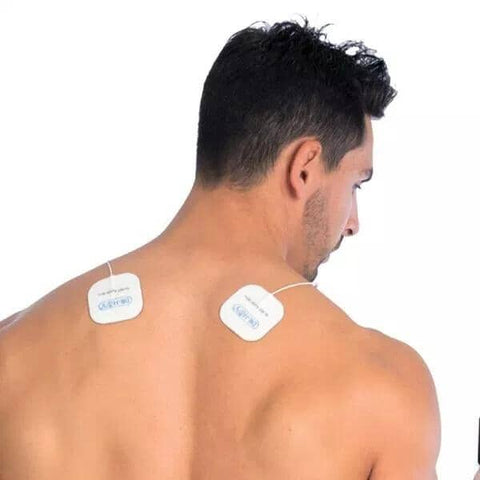
Dr. Ho Pain Management
Find relief and manage your pain effectively with the Dr. Ho Pain Management Collection at Showcase! Explore our range of innovative solutions, including the Circulation Promoter, Pain Therapy System, and Gel Pads. Dr. Ho's pain management products are designed to improve circulation and provide targeted therapy to alleviate discomfort and pain. With over 150 locations in major malls across Canada and the United States, Showcase has been the Home of the Hottest Trends since 1994, including the latest in pain management technology. You can shop with confidence, whether you prefer to buy Dr. Ho's products online with same-day Doordash delivery or select our free shipping option with FedEx. Trust Showcase for the best pain management solutions, and experience a better, pain-free life with the Dr. Ho Pain Management Collection.